Pharmaflex
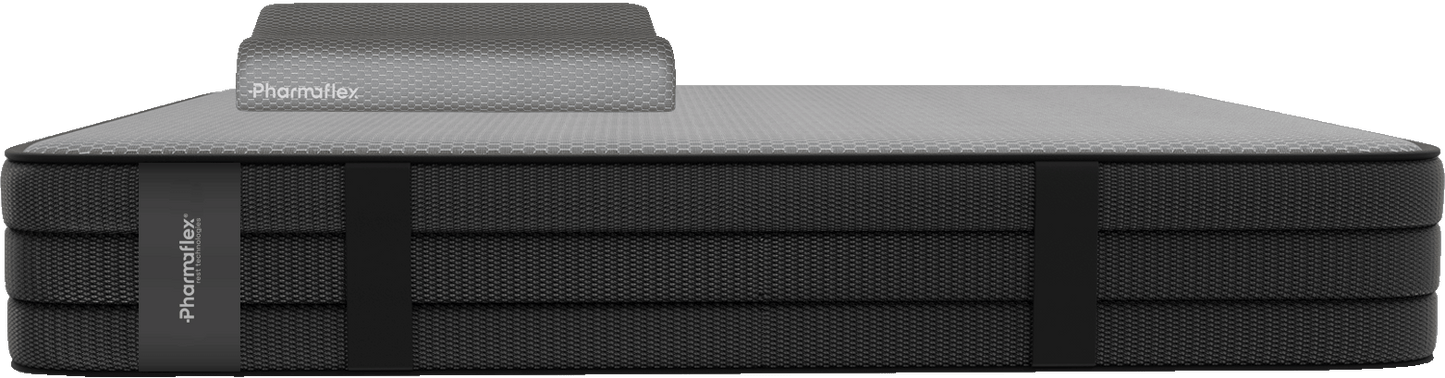
Bundle Materasso Performance Hybrid + Cervical (Risparmi 20€)
Bundle Materasso Performance Hybrid + Cervical (Risparmi 20€)
Regular price
€608,90 EUR
Regular price
Sale price
€608,90 EUR
Taxes included.
Shipping calculated at checkout.
Quantity
Couldn't load pickup availability
Bundle: Materasso Perfomance Hybrid + Cervical